What is Transarterial Chemoembolization (TACE)?
TACE is a specialized, minimally invasive procedure most commonly used to treat liver cancer (Hepatocellular Carcinoma, or HCC). It is performed by an Interventional Radiologist and combines two powerful treatments: targeted chemotherapy delivery and vessel embolization (blocking).
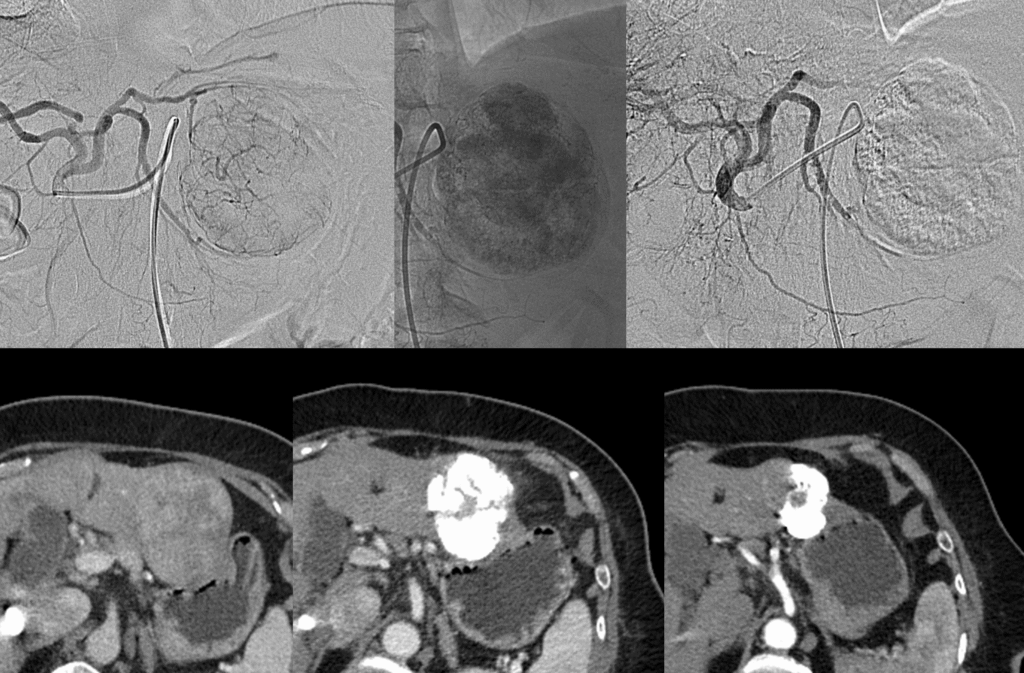
Key Benefits of TACE (Why is this procedure needed?)
TACE is often the standard of care for patients with liver cancer who are not candidates for surgery or transplantation, offering powerful local control.
| Benefit | Description |
|---|---|
| Targeted High-Dose Therapy | Delivers chemotherapy directly to the tumor site. This local delivery allows for a significantly higher drug concentration in the cancer cells compared to standard systemic chemotherapy, while reducing whole-body side effects. |
| Tumor Control | The procedure aims to stop the tumor from growing or cause it to shrink (objective response rate is typically around 50%−70%), which can extend the patient’s survival. |
| Minimally Invasive | Requires only a small puncture site (like a large needle stick) leading to much less trauma, minimal scarring, and a significantly faster recovery compared to open surgery. |
| Bridge to Transplant/Surgery | Can be used to keep a tumor controlled (shrunken) while a patient waits for a liver transplant or to make a tumor operable for a subsequent surgical resection. |
Understanding the Risks and Potential Complications
TACE is generally considered safe, but because it involves both arterial access and the delivery of chemotherapy and particles, it carries specific risks that your interventional radiology team will discuss with you.
General and Frequent Complication Rates
| Complication Type | Incidence (Likelihood) | Details |
|---|---|---|
| Post-Embolization Syndrome (PES) | High (Commonly affects 60%−80% of patients) | A temporary, flu-like condition caused by the inflammatory response to the treated, dying tumor tissue. Symptoms include fever, pain in the upper right abdomen, nausea, and vomiting, typically lasting 1 to 7 days and managed effectively with medication. |
| Minor Site Complications | High (Common) | Bruising, mild pain, or swelling (hematoma) at the catheter insertion site (groin/wrist). This usually resolves within a few days to weeks. |
| Allergic Reaction to Dye | Uncommon (<1 in 100 people) | Mild reactions (itching, rash) are rare. Severe allergic reactions (anaphylaxis) are extremely rare. |
Specific and Serious Risks
| Complication Type | Incidence (Likelihood) | Details |
|---|---|---|
| Major Procedure-Related Event | Low (Approx. 1%−3% per patient) | The rate of major events requiring intervention (e.g., severe hemorrhage, liver abscess, or vascular damage) is very low. |
| Liver Function Deterioration/Failure | Rare | TACE can temporarily stress the liver. In patients with severe pre-existing liver disease (cirrhosis), there is a risk of acute liver failure. This is why liver function must be carefully assessed before TACE. |
| Non-Target Delivery | Rare | The chemo-embolic mixture may inadvertently travel to healthy parts of the liver or other organs (e.g., the gallbladder, stomach), causing localized tissue damage, ulceration, or cholecystitis (gallbladder inflammation). |
| Treatment-Related Mortality (TRM) | Very Low (Approximately 1%−4%) | The risk of death directly related to the procedure is very low and is almost always associated with patients who have advanced liver disease or large, aggressive tumors. |
| Infection/Abscess | Very Rare (<0.1% per procedure) | Infection in the liver (abscess) can occur, especially in patients who have had prior surgery involving the bile ducts. This requires aggressive antibiotic treatment. |
Bibliography
- Cleveland Clinic. “Transarterial Chemoembolization (TACE): Definition & Procedure.” Cleveland Clinic, 2022.
- RadiologyInfo.org. “TACE Cancerous Tumor Therapy.” Radiological Society of North America (RSNA), 2021.
- Cancer.Net. “Transarterial Chemoembolization (TACE) for Liver Cancer.” American Society of Clinical Oncology (ASCO), 2023.
